Search Results for " Cefdinir "
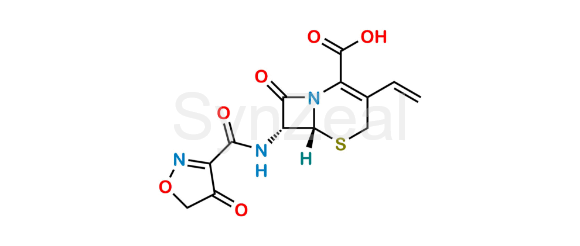
Cefdinir Isoxazole Analog (USP)
| SZ CAT No: | SZ-C020004 |
| CAS No | 1356842-10-8 |
| Mol.F. | C13H11N3O6S |
| Mol.Wt. | 337.3 |
| Inv. Status | Under QC Approval |
Chemical Name: (6R,7R)-7-(4-Hydroxyisoxazole-3-carboxamido)-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (as per USP)
Shipping Temperature: Ambient
HSN Code: 38229010
Country of Origin: India
Smiles: O=C(C(N12)=C(C=C)CS[C@]2([H])[C@H](NC(C3=NOCC3=O)=O)C1=O)O
Cefdinir Isoxazole Analog (USP) is chemically (6R,7R)-7-(4-Hydroxyisoxazole-3-carboxamido)-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (as per USP). Cefdinir Isoxazole Analog (USP) is supplied with detailed characterization data compliant with regulatory guideline. Cefdinir Isoxazole Analog (USP) can be used for the analytical method development, method validation (AMV), Quality Controlled (QC) application for Abbreviated New Drug Application (ANDA) or during commercial production of Cefdinir.
The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. SynZeal products are for analytical purpose only and not for human use.
Related products
Disclaimer
SynZeal product information given on this website is as per the existing understanding while publishing the details on website. The customer is responsible for assessing the accuracy of the information at the time of actual purchase.
SynZeal will update these details as per new development or finding in product specification without further noticed.

