OUR BLOGS
Check out our latest and upcoming Blogs.
Contact Us.png)
July 26 2023
We are excited to announce that SynZeal will be exhibiting at CPhI Worldwide Barcelona 2023 – the pharmaceutical industry's premier event! 📅 Event Details: 📍 Location: Fira de Barcelona, Barcelo...
Read More ›
July 26 2023
SynZeal is delighted to announce participation as an exhibitor at Analitica Latin America 2023, the premier event for laboratory technology and analytical science, to be held in São Paulo, Braz...
Read More ›.png)
May 5 2022
Fluocinonide is a synthetic glucocorticoid and derivative of fluocinolone acetonide with anti-inflammatory and antipruritic activities. Fluocinonide is a potent glucocorticoid used topically as an anti-inflammatory agent for the treatment of skin disorders such as eczema and seborrhoeic dermatitis.
Read More ›.png)
April 28 2022
Fluocinolone binds the glucocorticoid receptor, followed by translocation of the ligand-receptor complex to the nucleus and transcription activation of genes containing glucocorticoid-respons...
Read More ›
April 22 2022
Fesoterodine also known as toviaz, belongs to the class of organic compounds known as diphenylmethanes. Fesoterodine is a competitive muscarinic receptor antagonist with muscle relaxant and uri...
Read More ›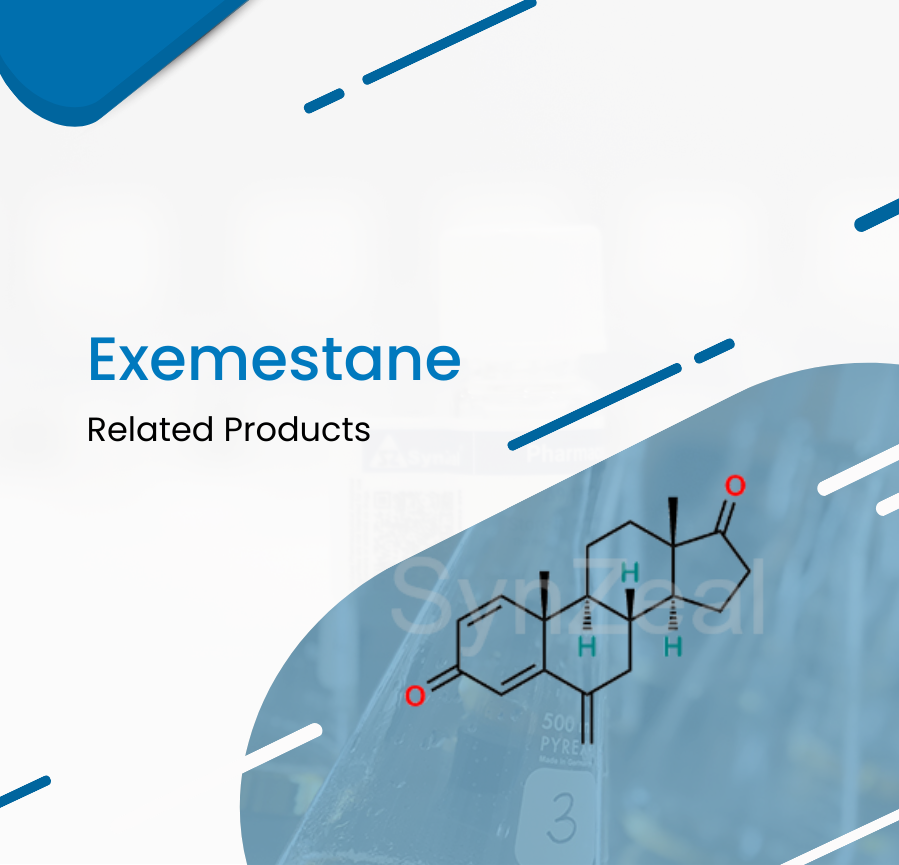
April 14 2022
Exemestane binds irreversibly to and inhibits the enzyme aromatase, thereby blocking the conversion of cholesterol to pregnenolone and the peripheral aromatization of androgenic precursors into...
Read More ›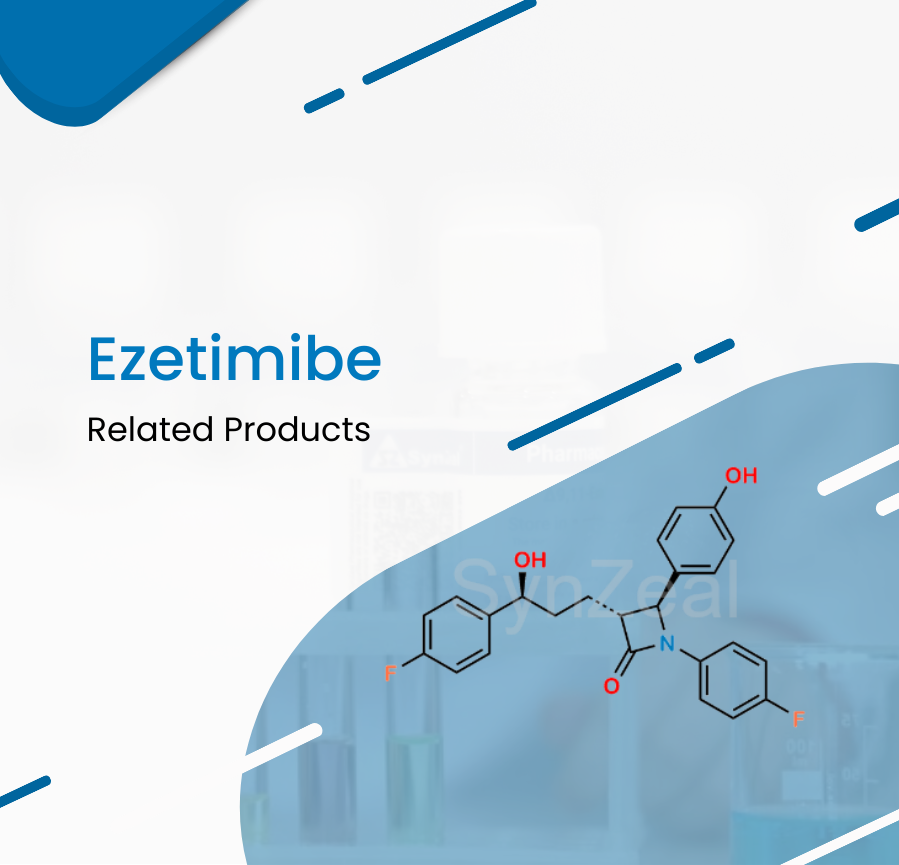
April 8 2022
Ezetimibe is a lipid-lowering compound that inhibits intestinal cholesterol and phytosterol absorption. Ezetimibe is used as an adjunctive therapy to a healthy diet to lower cholesterol levels in prim...
Read More ›
March 31 2022
Etonogestrel molecule is a 3-ketodesogestrel or 19-nortestosterone which is a synthetic biologically active metabolite of progestin desogestrel.Etonogestrel is a drug which is used for use as a female...
Read More ›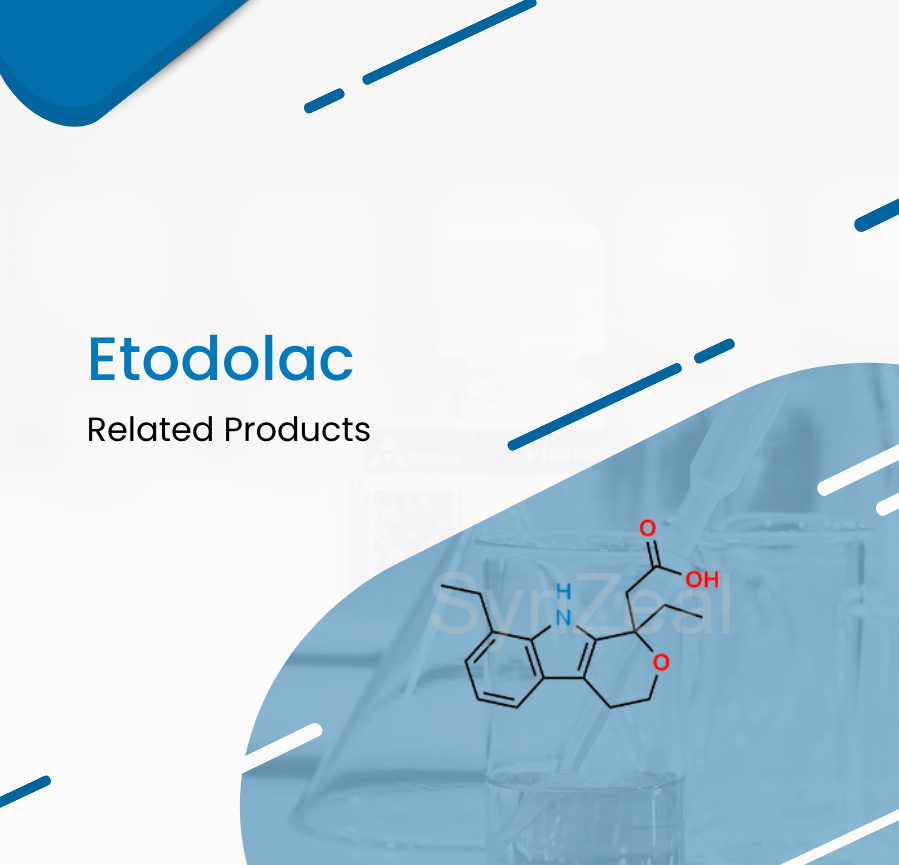
March 24 2022
Etodolac is a pyranocarboxylic acid and non-steroidal anti-inflammatory drug (NSAID) with antipyretic and analgesic activities. Etodolac used for the management of mild to moderate pain, fever, a...
Read More ›
March 16 2022
Ethinylestradiol is a semisynthetic estrogen. Ethinyl estradiol binds to the estrogen receptor complex and enters the nucleus, activating DNA transcription of genes involved in estrogenic cellular res...
Read More ›