- categories.breadcrumb.top /
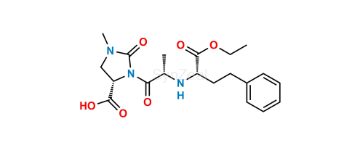
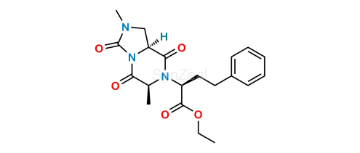
- Imidapril
- A
- B
- C
- D
- E
- F
- G
- H
- I
- J
- K
- L
- M
- N
- O
- P
- Q
- R
- S
- T
- U
- V
- W
- X
- Y
- Z
Imidapril Related Products
SynZeal is a leading innovator in pharmaceutical reference standards. We supply high-quality Reference Standards of Imidapril, pharmacopeial and non-pharmacopeial Imidapril impurities, metabolites, stable isotope products, and nitrosamines (N-NO products). Imidapril impurities reference standards are useful in pharmaceutical research. They are useful in product development, ANDA and DMF filing, quality control (QC), method validation, and stability studies. It is also useful in the identification of unknown impurities and the assessment of genotoxic potential.
Imidapril-related products are thoroughly characterized. Imidapril products are supplied with detailed COA & analytical data meeting regulatory compliance. We can also provide EP/USP traceable standards based on your requirements. The supplied products are re-tested at regular intervals.
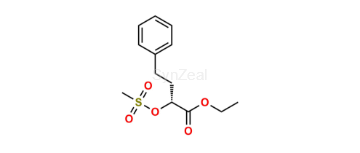
Imidapril
| SZ目录编号: | : SZ-I057001 |
| CAS 号 | : 89371-37-9 |
| 分子式 | : C20H27N3O6 |
| 分子量 | : 405.5 |
| 投资状态 | : Custom Synthesis |
| Rel. CAS No | : 89396-94-1 (HCl salt) |
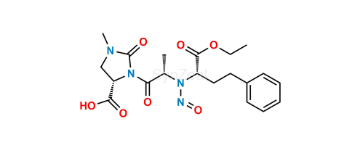
Imidapril Impurity 2
| SZ目录编号: | : SZ-I057004 |
| CAS 号 | : 1470364-43-2 |
| 分子式 | : C20H25N3O5 |
| 分子量 | : 387.4 |
| 投资状态 | : Custom Synthesis |
- Abiraterone Acetate
- Alfuzosin
- Amiodarone
- Amlodipine
- Anastrozole
- Aripiprazole
- Bisoprolol
- Bupropion
- Carbamazepine
- Chlorphenamine
- Clotrimazole
- Clozapine
- Colesevelam
- Darifenacin
- Dienogest
- Dofetilide
- Domperidone
- Donepezil
- Doxazosin
- Doxylamine
- Drospirenone
- Duloxetine
- Efavirenz
- Erythromycin
- Escitalopram
- Esomeprazole
- Estradiol Valerate
- Ethacrynic Acid
- Fesoterodine
- Fluocinonide
- Formoterol
- Fulvestrant
- Gabapentin
- Griseofulvin
- Haloperidol Decanoate
- Isoniazide
- Lercanidipine
- Letrozole
- Levetiracetam
- Lopinavir
- Marbofloxacin
- Melphalan
- Memantine
- Metronidazole
- Modafinil
- Mycophenolate Mofetil
- Nadolol
- Nateglinide
- Nicardipine
- Nifedipine
- Olanzapine
- Omeprazole
- Ondansetron
- Oseltamivir
- Paliperidone
- Pantoprazole
- Paracetamol
- Perphenazine
- Phentolamine
- Pramipexole
- Progesterone
- Propafenone
- Propranolol
- Rabeprazole
- Ramipril
- Ranitidine
- Ritonavir
- Rizatriptan
- Ropinirole
- Sertraline
- Tacrolimus
- Telmisartan
- Tinidazole
- Tryptophan
- Vilazodone
- Zidovudine
- Acebutolol
- Acetylcysteine
- Aciclovir
- Albendazole
- Ambrisentan
- Ampicillin
- Ascomycin
- Atenolol
- Atomoxetine
- Atorvastatin
- Pharmaceutical Reference Standards
- Azacitidine
- Bosentan
- Budesonide
- Canagliflozin
- Carvedilol
- Cefalexin
- Cefdinir
- Cetirizine
- Cimetidine
- Citalopram
- Clarithromycin
- Clavulanate
- Clonidine
- Clopidogrel
- Cyclobenzaprine
- Cyclosporin
- Cyproterone
- Dapagliflozin
- Daptomycin
- Dasatinib
- Decitabine
- Deferasirox
- Desloratadine
- Desvenlafaxine
- Dexamethasone
- Diacerein
- Diclofenac
- Diltiazem
- Dipyridamole
- Docetaxel
- Dorzolamide
- Dutasteride
- Empagliflozin
- Emtricitabine
- Enalapril
- Enrofloxacin
- Entacapone
- Entecavir
- Eprosartan
- Erlotinib
- Everolimus
- Ezetimibe
- Febuxostat
- Felodipine
- Finasteride
- Fluconazole
- Fluticasone
- Fosaprepitant
- Gatifloxacin
- Gemcitabine
- Glimepiride
- Glipizide
- Hydrochlorothiazide
- Iloperidone
- Irbesartan
- Irinotecan
- Ketoconazole
- Ketoprofen
- Ketorolac
- Lamivudine
- Lamotrigine
- Levodopa
- Levofloxacin
- Levonorgestrel
- Levothyroxine
- Lidocaine
- Linagliptin
- Linezolid
- Losartan
- Meloxicam
- Metformin
- Methotrexate
- Methyldopa
- Metoprolol
- Micafungin
- Milbemycin
- Mirtazapine
- Mitomycin
- Mometasone
- Montelukast
- Moxifloxacin
- Naproxen
- Nilotinib
- Norethindrone
- Norethindrone Acetate
- Norfloxacin
- Ofloxacin
- Olmesartan
- Olopatadine
- Orlistat
- Palonosetron
- Paroxetine
- Pazopanib
- Pemetrexed
- Perindopril
- Pitavastatin
- Prasugrel
- Pravastatin
- Prazosin
- Prednisolone
- Pregabalin
- Raloxifene
- Riociguat
- Risperidone
- Rivaroxaban
- Rivastigmine
- Roflumilast
- Rosuvastatin
- Salmeterol
- Sildenafil
- Silodosin
- Simvastatin
- Solifenacin
- Sorafenib
- Sumatriptan
- Tadalafil
- Tamsulosin
- Temsirolimus
- Tenofovir
- Terazosin
- Tiotropium
- Tolterodine
- Ursodeoxycholic
- Valaciclovir
- Vancomycin
- Vandetanib
- Vardenafil
- Venlafaxine
- Voriconazole
- Warfarin
- Zolmitriptan
- Daclatasvir
- Atovaquone
- Fenofibrate
- Atropine
- Lansoprazole
- Loratadine
- Nevirapine
- Quetiapine
- Temozolomide
- Valsartan
- Spironolactone
- Trazodone
- Betamethasone
- Hydrocortisone
- Testosterone
- Tetrabenazine
- Ravuconazole
- Isavuconazole
- Efinaconazole
- Ticagrelor
- Azelastine
- Amitriptyline
- Doxepin
- Sitagliptin
- Imatinib
- Ciprofloxacin
- Brimonidine
- Lisinopril
- Amphotericin B
- Aspirin
- Urapidil
- Acetazolamide
- Amoxicillin
- Atazanavir
- Exemestane
- Galantamine
- Ibuprofen
- Mesalazine
- Labetalol
- Salbutamol
- Adapalene
- Aceclofenac
- Agomelatine
- Mifepristone
- Tibolone
- Methylprednisolone
- Azithromycin
- Sofosbuvir
- Clobetasol Propionate
- Clobetasone Butyrate
- Chlorambucil
- Lenalidomide
- Ledipasvir
- Velpatasvir
- Fluoxetine
- Dabigatran
- Amisulpride
- Aprepitant
- Candesartan
- Betahistine
- Buspirone
- Celecoxib
- Azilsartan
- Bendamustine
- Chlortalidone
- Cinacalcet
- Sunitinib
- Ramelteon
- Bortezomib
- Palbociclib
- Teneligliptin
- Tofacitinib
- Pioglitazone
- Paclitaxel
- Saxagliptin
- Ibrutinib
- Epalrestat
- Colchicine
- Tizanidine
- Bromhexine
- Albuterol
- Acetophenone
- Drotaverine
- Acetovanillone
- Fluvoxamine
- Fosphenytoin
- Mebendazole
- Capecitabine
- Hydroxychloroquine
- Enzalutamide
- Eletriptan
- Mexiletine
- Amantadine
- Nicorandil
- Clomipramine
- Raltegravir
- Algestone
- Ampyrone
- Atracurium
- Frovatriptan
- Benzoic Acid
- Benzonatate
- Brexpiprazole
- Carebastine
- Captopril
- Desonide
- Cefazolin
- Cevimeline
- Cinchocaine
- Clioquinol
- Cyproheptadine
- Cytidine
- Ebastine
- Fampridine
- Felbamate
- Fosinopril
- Gliclazide
- Guaifenesin
- Guanine
- Hydroquinone
- Indapamide
- Mercaptopurine
- Metaxalone
- Metoclopramide
- Etodolac
- Niacin
- Nicotine
- Nimodipine
- Nizatidine
- Oxcarbazepine
- Penicillin
- Phenoxybenzamine
- Pitofenone
- Pivmecillinam
- Pyridoxal
- Ranolazine
- Sulfacetamide sodium
- Sulfamethoxazole
- Teriflunomide
- Timolol
- Tolnaftate
- Topiramate
- Valproic Acid
- Zoledronic acid
- Gentamicin
- Moxonidine
- Isoproterenol
- Triamcinolone Acetonide
- Estradiol Enanthate
- Prilocaine
- Flecainide
- Posaconazole
- Pregnenolone
- Delafloxacin
- Ganciclovir
- Cytosine
- Estrone
- Etoricoxib
- Fluphenazine
- Glibenclamide
- Heparin
- Nisoldipine
- Oxolamine
- Oxybutynin
- Pimecrolimus
- Pyridoxine
- Roxithromycin
- Mesna
- Fluvastatin
- Miscellaneous
- Glutamic Acid
- Nebivolol
- Brinzolamide
- Cyanocobalamin
- Desoximetasone
- Nepafenac
- Fexofenadine
- Dacarbazine
- Valganciclovir
- Trifluoperazine
- Abacavir
- Acesulfame Potassium
- Amoxapine
- Pirfenidone
- Cholesterol
- Oxytetracycline
- Fenticonazole Nitrate
- Apremilast
- Ropivacaine
- Zopiclone
- Cabazitaxel
- Ornidazole
- Tranexamic Acid
- Varenicline
- Estradiol
- Avanafil
- Alogliptin
- Triamcinolone
- Cabergoline
- Pyridine
- Sulpiride
- Ivabradine
- Bisacodyl
- Promethazine
- Cariprazine
- Ivacaftor
- Eslicarbazepine acetate
- Neostigmine
- Difluprednate
- Rapamycin
- Phentermine
- Lacosamide
- Clofarabine
- Chlorpromazine
- Estradiol Hemihydrate
- Haloperidol
- Cefadroxil
- Equilin
- Equilenin
- Iminostilbene
- Dapsone
- Gestodene
- Mirabegron
- Caffeine
- Fosfomycin
- Rupatadine
- Loteprednol
- Darunavir
- Afatinib
- Chlormadinone
- Bepotastine
- Lurasidone
- Famotidine
- Fingolimod
- Diosmin
- Epinephrine
- Mivacurium
- Minocycline
- Tizoxanide
- Estriol
- Triamterene
- Furosemide
- Ulipristal
- Etravirine
- Prednisone
- Nintedanib
- Nimesulide
- Osimertinib
- Amodiaquine
- Anthracene
- Trimethoprim
- Penciclovir
- Camptothecin
- Chloroquine
- Rifaximin
- Regadenoson
- Penicillamine
- Epinastine
- Bromfenac
- Testosterone Cypionate
- Bilastine
- Macitentan
- Dropropizine
- Vecabrutinib
- Famciclovir
- Bicalutamide
- Carisoprodol
- Sacubitril
- Allylestrenol
- Caspofungin
- Ginkgolide
- Kaempferol
- Quercetin
- Piperaquine
- Artemisinin
- Vortioxetine
- Lapatinib
- Cefaclor
- Tetracaine
- Eltrombopag
- Indomethacin
- Chlorhexidine
- Vigabatrin
- Permethrin
- Rilpivirine
- Edoxaban
- Bendroflumethiazide
- Trimetazidine
- Levosulpiride
- Orphenadrine
- Eliglustat
- Desipramine
- Aminocaproic Acid
- Lenvatinib
- Tolcapone
- Doxycycline
- Tamoxifen
- Carfilzomib
- Tolvaptan
- Halobetasol
- Fluocinolone
- Clomiphene
- Halcinonide
- Aspartame
- Trimipramine
- Cyclophosphamide
- Cilastatin
- Deracoxib
- Metolazone
- Meclizine
- Mitoxantrone
- Metamizole
- Octisalate
- Apixaban
- Parecoxib
- Folinic Acid
- Isometheptene
- Cholecalciferol
- Glycopyrrolate
- Edaravone
- Alvimopan
- Flurbiprofen
- Azathioprine
- Bezafibrate
- Meropenem
- Piperacillin
- Gefitinib
- Latanoprost
- Dolutegravir
- Hyoscine Butylbromide
- Carbidopa
- Orciprenaline
- Nomegestrol
- Anagrelide
- Rasagiline
- Vildagliptin
- Baricitinib
- Brivaracetam
- Terbutaline
- Thiothixene
- Verapamil
- Flunixin
- Cefoperazone
- Venetoclax
- Dantrolene
- Carbinoxamine
- Tiamulin
- Ornithine
- Levocarnitine
- Ifosfamide
- Prochlorperazine
- Ivermectin
- Benzocaine
- Misoprostol
- Amikacin
- Alverine
- Clindamycin
- Nifuratel
- Sotalol
- Molsidomine
- Pyrantel
- Promazine
- Diphenhydramine
- Sibutramine
- Nitrofurantoin
- Propofol
- Cilnidipine
- Rifapentine
- Piracetam
- Thiamine
- Glucosamine
- Etilefrine
- Loperamide
- Ipratropium
- Fluphenazine Decanoate
- Granisetron
- Bumetanide
- Simethicone
- paraben
- Tigecycline
- Hydroxyzine
- Minoxidil
- Selexipag
- Pomalidomide
- Doxorubicin
- Epirubicin
- Daunorubicin
- Amorolfine
- Spiramycin
- Trandolapril
- Cefixime
- Abamectin
- Mebeverine
- Leflunomide
- Tasimelteon
- Fluorometholone
- Ceftriaxone
- Desogestrel
- Praziquantel
- Zileuton
- Phenylephrine
- Etonogestrel
- Cloxacillin
- Crisaborole
- Sulbactam
- Droxidopa
- Cefuroxime
- Cefalotin
- Busulfan
- Amiloride
- Mupirocin
- Thiocolchicoside
- Repaglinide
- Cobicistat
- Nitazoxanide
- Evogliptin Tartrate
- Pimavanserin
- Asenapine
- Trimebutine
- Dydrogesterone
- Cefpodoxime Proxetil
- Dosulepin
- Luteolin
- Vitexin
- Riluzole
- Prulifloxacin
- Isotretinoin
- Rufinamide
- Fenoprofen
- Dexamethasone sodium phosphate
- Idarubicin
- Polmacoxib
- Aztreonam
- Flucytosine
- Fenoterol
- Pharmaceutical Reference Standards
- Dicycloverine
- Allopurinol
- Adefovir
- Calanolide
- Carbovir
- Cabotegravir
- Chicoric Acid
- Mildronate
- Ethinyl Estradiol
- Atripla
- Bevirimat
- Ribociclib
- Riboflavin
- Manidipine
- Sapropterin
- Methacycline
- Phytonadione
- Dimenhydrinate
- Neamine
- Folic Acid
- Levocetirizine
- Retinol
- Tocopheryl acetate
- Biotin
- Bupivacaine
- Clonixin
- Sparfloxacin
- Cycloserine
- Imidocarb Dipropionate
- Rocuronium
- Linolenic Acid
- Methionine
- Flucloxacillin
- Citicoline
- Abemaciclib
- Niraparib
- Acalabrutinib
- Brigatinib
- Cobimetinib
- Carprofen
- Dexpanthenol
- Sugammadex
- Thioctic Acid
- Tiagabine
- Menthone
- Menthol
- Isopulegol
- Ascorbic Acid
- Piperidine
- Nabumetone
- Acedoben
- Sebacic acid
- Ephedrine hydrochloride
- Cefepime
- Luliconazole
- Ertapenem
- Glycopyrronium bromide
- Mosapride
- Silibinin
- Obeticholic Acid
- Clobutinol Hydrochloride
- Etomidate
- Midostaurin
- Alectinib
- Bictegravir
- Oxaprozin
- Bleomycin
- Secnidazole
- Fluoxymesterone
- Pentoxifylline
- Chlorhexidine Gluconate Solution
- Roxatidine
- Fluorouracil
- Hycosamide
- Demeclocycline
- Cladribine
- Axitinib
- Dronedarone Hydrochloride
- Milrinone
- Midodrine Hydrochloride
- Ramosetron
- Itopride
- Oxitropium bromide
- Racecadotril
- Neomycin sulfate
- Loxoprofen
- Valbenazine
- Gemfibrozil
- Alcaftadine
- Baclofen
- Lymecycline
- Lorcaserin
- Amfenac
- Oxomemazine
- Diflorasone Diacetate
- Dapoxetine
- Methocarbamol
- Cefazedone
- Macimorelin Acetate
- Ertugliflozin
- Netarsudil
- Ozenoxacin
- Letermovir
- Copanlisib
- Enasidenib
- Neratinib
- Betrixaban
- Naldemedine
- Safinamide
- Telotristate ethyl
- Etelcacetide
- Prucalopride
- Gilteritinib
- Larotrectinib
- Glasdegib
- Revefenacin
- Lorlatinib
- Baloxavir marboxil
- Talazoparib
- Dacomitinib
- Doravirine
- Eravacycline
- Stiripentol
- Migalastat
- Lusutrombopag
- Elagolix sodium
- Tafenoquine
- Ivosidenib
- Tecovirimat
- Encorafenib
- Binimetinib
- Plazomicin
- Ruxolitinib
- Avatrombopag
- Lofexidine
- fosnetupitant
- Fostamatinib
- Tezacaftor
- Triclabendazole
- Solriamfetol
- Siponimod
- Erdafitinib
- Alpelisib
- Bremelanotide
- Selinexor
- Ferric Maltol
- Relebactam
- Apalutamide
- Almotriptan
- Mepyramine maleate
- Clidinium Bromide
- Benazepril hydrochloride
- Cinnarizine
- Phenytoin
- Bromopride
- Cilostazol
- Ceftazidime pentahydrate
- Nifuroxazide
- Naratriptan
- Enalaprilat dihydrate
- Flumethasone
- Hydroxocobalamin Acetate
- Lincomycin Hydrochloride
- Clofazimine
- Orbifloxacin
- Doxofylline
- Flibanserin
- Medetomidine
- Metaraminol
- Cabozantinib
- Sulfasalazine
- Indoramin
- Otilonium Bromide
- Thioridazine
- Eriocitrin
- Hesperetin
- Lumefantrine
- Levomepromazine
- Digoxin
- Vorapaxar
- Iopamidol
- Dimethoate
- Sulphanilamide
- Pyrazinamide
- Pyrimethamine
- Atosiban
- Creatinine
- Betaxolol
- Dolasetron
- Tiopronin
- Molindone
- Tirofiban
- Piroxicam
- Dihydrostreptomycin
- Isomaltitol
- Acemetacin
- Rifampicin
- Vaborbactam
- Methenolone Acetate
- Oritavancin
- Ciprofibrate
- Calcipotriol
- Etoposide
- Mefenamic Acid
- Nebrosamine
- Tobramycin
- Tazarotene
- Tryptophol
- Plerixafor
- Moxidectin
- Isoflurane
- Sevoflurane
- Quinoline
- Icatibant
- Tropicamide
- Artemether
- 2,4-Dichlorobenzyl alcohol
- Proguanil
- Rapidosept
- Levodropropizine
- Disulfiram
- Flupentixol
- Pretomanid
- Diroximel Fumarate
- Acarbose
- Cefprozil Monohydrate
- Ketotifen Fumarate
- Sucralfate
- Lornoxicam
- Echimidine
- Erucifoline
- Europine
- Heliotrine
- Intermedine
- Jacobine
- Lasiocarpine
- Lycopsamine
- Monocrotaline
- Retrorsine
- Senecionine
- Seneciphylline
- Senecivernine
- Senkirkine
- Trichodesmine
- Tebipenem Pivoxil
- Eribulin
- Glutamine
- Torasemide
- Alimemazine Hemitartrate
- Isosorbide Mononitrate
- Betamethasone Sodium Phosphate
- Tretinoin
- Vecuronium Bromide
- Melatonin
- Carglumic Acid
- Acotiamide
- Levalbuterol
- Tazobactam
- Risedronic Acid
- Ingenol
- Maprotiline Hydrochloride
- Ergocalciferol
- Idelalisib
- Ormeloxifene
- Balsalazide
- Leuprolide
- Acrivastine
- Pharacine
- Tegaserod
- Betaine
- Benfotiamine
- Adiphenine
- Brompheniramine Maleate
- Delamanid
- Nystatin
- Megestrol Acetate
- Pentetreotide
- Carmustine
- Flumazenil
- Acebrophylline
- Doxorubicinol
- Primapterin
- Benzoyl Peroxide
- Sennoside
- Perampanel
- Lubiprostone
- Norepinephrine
- Adrenalone
- Phenacetin
- Eberconazole
- Bosutinib
- Maropitant
- Zonisamide
- Meglumine
- Propyl Gallate
- Carboplatin
- Oxaliplatin
- Calcitriol
- Fructose
- Carboprost Tromethamine
- Oleic Acid
- Eplerenone
- Benzbromarone
- Lactitol
- Alendronate
- Fludrocortisone
- Naphazoline
- Bimatoprost
- Carbocisteine
- Econazole
- Tafamidis
- Miconazole
- Tavaborole
- Trientine
- Gabalactam
- Cetrorelix
- Cisplatin
- Flubendazole
- Butoconazole
- Remdesivir
- Travoprost
- Relugolix
- Treprostinil
- Isoxsuprine
- Cefoxitin
- Cefotaxime Sodium
- Firocoxib
- Hyoscyamine
- Nadifloxacin
- Ceramide
- Favipiravir
- Benserazide
- Benzyl Benzoate
- Clevidipine
- Picosulfate Sodium
- Quinapril
- Glycocholic Acid
- Benzydamine
- Elmustine
- Lacidipine
- Narirutin
- Ticlopidine
- Nortriptyline
- Detomidine
- Ixabepilone
- Calcobutrol
- Tetracycline
- Roxadustat
- Lifitegrast
- Pinaverium
- Ethosuximide
- Nefopam
- Dimetindene
- Cisatracurium
- Lactulose
- Selegiline
- Goserelin
- Nitrosamines
- Dequalinium Chloride
- Levocabastine Hydrochloride
- Regorafenib
- Tolperisone
- Levomefolate
- Rifabutin
- Celiprolol Hydrochloride
- Tranylcypromine
- Rucaparib
- Thiotepa
- Pralatrexate
- Uridine
- Terbinafine
- Acitretin
- Flavoxate Hydrochloride
- Methazolamide
- Phenazopyridine
- Noradrenolone
- Hydralazine
- Isosorbide Dinitrate
- Ternidazole
- Ribavirin
- Adenosine
- Dexlansoprazole
- Sulfadoxine
- Noradrenaline
- Fosamprenavir
- Benidipine
- Maraviroc
- Acetyltributyl Citrate
- Pridinol
- Adrenaline
- Tolfenamic acid
- Calcifediol
- Bexarotene
- Pyridostigmine
- Diaveridine
- Vasopressin
- Sulfadiazine
- Naringenin
- Nicergoline
- Cinchonidine
- Quinidine
- Prednisolone Sodium Phosphate
- Triricinolein
- Butylhydroxyanisole
- Promestriene
- Primidone
- Nandrolone Decanoate
- Deflazacort
- Clemastine
- Elobixibat
- Terephthalic acid
- Nitenpyram
- Phloroglucinol
- Benzalkonium Chloride
- Ridinilazole
- Ceritinib
- Pilocarpine
- Nelarabine
- Ganirelix
- Milnacipran
- Zafirlukast
- Probenecid
- Oxymetazoline
- Etofenamate
- Proparacaine
- Deltamethrin
- Droperidol
- Nitisinone
- Corticosterone
- Meprednisone
- Didanosine
- Esmolol
- Lafutidine
- Flupirtine
- Bazedoxifene
- Acamprosate
- Lovastatin
- Tribenoside
- Barnidipine
- Phenylalanine
- Tulathromycin
- Propantheline Bromide
- Oxyquinoline Sulfate
- Brivudine
- Ethambutol
- Thiamazole
- Gemifloxacin
- Sisomicin Sulfate
- Butropium Bromide
- Levamisole
- Pimobendan
- Dalbavancin
- Trimethobenzamide
- Linaclotide
- Liothyronine
- Levosimendan
- Indacaterol
- Cromolyn
- Mianserin
- Tapentadol
- Mepivacaine
- Suvorexant
- Gadobutrol
- Pyridoxamine
- Apramycin
- Nebramine
- Lobeglitazone
- Bethanechol
- Toxaphene
- Cortexolone
- Ilaprazole
- Selamectin
- Flutamide
- Phenyramidol
- Zephirol
- Benzenesulfonic Acid
- Gadoteridol
- Isosulfan Blue
- Cilazapril
- Naftifine
- Hexamidine
- Methenamine
- Abietic Acid
- Pranlukast
- Mertansine
- Methoxsalen
- Fluprednidene Acetate
- Cefditoren Pivoxil
- Faropenem
- Octenidine
- Metopimazine
- Antipyrine
- Bifonazole
- Estradiol Benzoate
- Triptorelin
- Monensin
- Miglustat
- Dicloxacillin
- Azilsartan Medoxomil
- Dobutamine
- Colestipol
- Norgestimate
- Olaparib
- Imipramine
- Laidlomycin
- Argatroban
- Vicenin
- Fedratinib
- Vonoprazan
- Diflucortolone Valerate
- Sucralose
- Benethamine penicillin
- Phenylbutazone
- Ciclesonide
- Chloramphenicol
- Octreotide
- Warburganal
- Desmosterol
- Guanfacine
- Upadacitinib
- Ceftiofur
- Fomepizole
- Phenylpropanolamine
- Molnupiravir
- Isothipendyl
- Methyl Prednisolone Suleptanate
- Procyclidine
- Gamithromycin
- Tafluprost
- Pentamidine
- Lufenuron
- Clodronate
- Hydroflumethiazide
- Altizide
- Reboxetine
- Tegoprazan
- Sultamicillin
- Fenoldopam
- Ceftaroline Fosamil
- Chenodeoxycholic acid
- Darolutamide
- Iloprost
- Beraprost
- Dinoprost
- Gemeprost
- Cloprostenol
- Dinoprostone
- Alprostadil
- Pamidronate
- Mefloquine
- Mevastatin
- Vilanterol
- Tivozanib
- Imipenem
- Boldenone Undecylenate
- Azelaic acid
- Ciclopirox
- Benzatropine
- Rebamipide
- Aprindine
- Levofolinate
- Corydaline
- Isoconazole
- Miriplatin
- Avermectin
- Crizotinib
- Rifamycin
- Valerenic Acid
- Doxercalciferol
- Pheniramine
- Xylometazoline
- Serotonin
- Dalfampridine
- Nedocromil
- Cidofovir
- Bempedoic Acid
- Deferiprone
- Diflunisal
- Elvitegravir
- Thiorphan
- Dexbrompheniramine
- Gemigliptin
- Nitrilotriacetic Acid
- Inositol
- Lysine
- Procainamide
- Fenspiride
- Cefotiam
- Fludarabine
- Novobiocin
- Remogliflozin
- Ibandronate
- Ibutilide
- Trihexyphenidyl
- Niclosamide
- Cefalonium
- Pantothenic Acid
- Umeclidinium Bromide
- Mutilin
- Dopamine
- Pralidoxime
- Parbendazole
- Azelnidipine
- Theophylline
- Ozanimod
- Dexrazoxane
- Penoxsulam
- Omadacycline
- Bisabolol
- Quinine
- Sorbitol
- Pirlimycin
- Terconazole
- Capsaicin
- Emetine
- Menaquinone
- Picaridin
- Norgestrel
- Nicotinamide
- Moclobemide
- Valdecoxib
- Anidulafungin
- Lupeol
- Desmopressin
- Romidepsin
- Sorbic Acid
- Scopolamine
- Tianeptine
- Dihydroartemisinin
- Bamifylline
- Diatrizoic Acid
- Flurandrenolide
- Cibenzoline
- Resveratrol
- Polyhexanide
- Gramicidin
- Cytarabine
- Amsacrine
- Pargeverine
- Apoatropine
- Levobunolol
- Perhexiline
- Protriptyline
- Cisapride
- Amtolmetin Guacil
- Cyclizine
- Cyamemazine
- Fosravuconazole
- Glecaprevir
- Pibrentasvir
- Zanubrutinib
- Lasmiditan
- Crotamiton
- Eperisone
- Amifampridine
- Cangrelor
- Trichlormethiazide
- Clobenzorex
- Buclizine
- Pyrvinium Pamoate
- Trabectedin
- Ochratoxin
- Aflatoxin
- Pericyazine
- Pipotiazine
- Zuclopenthixol
- Zuclopenthixol Decanoate
- Trospium Chloride
- Chlorprothixene
- Prednicarbate
- Ractopamine
- Buserelin
- Lanreotide
- Hesperidin
- Fluocortolone Pivalate
- Perazine Dimalonate
- Lynestrenol
- Beclomethasone
- Ipragliflozin
- Lumateperone
- Vericiguat
- Gimeracil
- Butenafine
- Tucatinib
- Bromocriptine Mesilate
- Trilostane
- Mecamylamine
- Aspartic Acid
- Histidine
- Arginine
- Tetryzoline Hydrochloride
- Cefozopran
- Cefacetrile
- Ceftizoxime Sodium
- Diquafosol
- Pelubiprofen
- Aminolevulinic Acid
- Metyrosine
- Vincristine Sulfate
- Difloxacin
- Alfacalcidol
- Voglibose
- Eptifibatide
- Gadodiamide
- Alclometasone Dipropionate
- Iopromide
- Cetylpyridinium Chloride
- Besifloxacin
- Rotigotine
- Avilamycin
- Phthalylsulfathiazole
- Toltrazuril
- Phenol
- Topiroxostat
- Methisoprinol
- Sulfadimethoxine
- Sulindac
- Meclofenamic Acid
- Erdosteine
- Vinblastine Sulfate
- Imiquimod
- Flunisolide
- Homatropine Hydrobromide
- Enoxolone
- Josamycin
- Itraconazole
- Clascoterone
- Zeaxanthin
- Eicosapentaenoic Acid
- Iohexol
- Toremifene
- Cefpirome Sulfate
- Cannabidiol
- Pirarubicin
- Primaquine
- Glatiramer Acetate
- Hexetidine
- Bedaquiline
- Biperiden Hydrochloride
- Imeglimin
- Phenoxyethanol
- Rutin
- Retinyl Palmitate
- Epoprostenol
- Propentofylline
- Cyantraniliprole
- Carbetocin
- Ponatinib
- Robenacoxib
- Zilpaterol
- Romifidine
- Acetylisovaleryltylosin
- Tioconazole
- Papaverine Hydrochloride
- Indinavir
- Eltrombopag Olamine
- Flunarizine
- Nirmatrelvir
- Vorinostat
- Telbivudine
- Chlorquinaldol
- Ceftobiprole
- Armodafinil
- Tocopherol
- Pitolisant
- Dinotefuran
- Fidaxomicin
- Maytansine
- Clenbuterol Hydrochloride
- Naftidrofuryl Oxalate
- Rimegepant
- Diclofenamide
- Meclocycline
- Umifenovir
- Malathion
- Pemigatinib
- Viloxazine
- Bemotrizinol
- Escin
- Glutathione
- Zofenopril
- Cyprodinil
- Flufentacet
- Florfenicol
- Thiamphenicol
- Vinflunine
- Dextromethorphan
- Eluxadoline
- Propiconazole
- Pendimethalin
- Docosahexaenoic Acid
- Dexamfetamine Sulfate
- Naloxone
- Peramivir
- Leucine
- Finerenone
- Carbazochrome
- Dobesilic Acid
- Chlorpropamide
- Methyl Salicylate
- Efonidipine
- Bromazepam
- Desoxycorticosterone Acetate
- Metribuzin
- Tebuconazole
- Atrazine
- Oxacillin
- Oxiracetam
- Curcumin
- Anthraquinone
- Pinoxaden
- Nortilidine
- Ubrogepant
- Fludrocortisone Acetate
- Oxprenolol
- Paricalcitol
- Avibactam
- Fenfluramine
- Irganox
- Imidapril
- Zolpidem
- Pindolol
- Tulobuterol
- Ubidecarenone
- Neotame
- Sodium Stearyl Maleate
- Cornigerine
- Prothioconazole
- Midazolam
- Bivalirudin
- Phenobarbital
- Dabrafenib
- Norelgestromin
- Tolmetin
- Codeinone
- Morphine
- Diethyltoluamide
- Amfepramone
- Cloperastine
- Codeine
- Morantel
- Fipronil
- Carbimazole
- Docusate
- Pseudoephedrine
- Ergotamine
- Ketamine Hydrochloride
- Levocloperastine Fendizoate
- Hidiosmin
- Ceftolozane
- Cefradine
- Ademethionine
- Pyraclostrobin
- Tropisetron
- Oxytocin
- Fentanyl
- Oxycodone
- Trametinib
- Harpagoside
- Methylergometrine
- Lisdexamfetamine
- Alprazolam
- Diazepam
- Methylphenidate
- Clobazam
- Naltrexone
- Tepotinib
- Oxazepam
- Lorazepam
- Flunitrazepam
- Baicalin
- Buprenorphine
- Apomorphine
- Tramadol
- Teferdin
- Chlorzoxazone
- Nordazepam
- Cinitapride
- Salcaprozate
- Clonazepam
- Resocortol
- Phenazepam
- Alosetron
- Thiamethoxam
- Diafenthiuron
- Plecanatide
- Indocyanine Green
- Bucindolol
- Trolamine
- Methylene Blue
- Chlordiazepoxide
- Febantel
- Esketamine Hydrochloride
- Ginsenoside
- Ripretinib
- Rosiglitazone
- Selpercatinib
- Zaleplon
- Spectinomycin
- Fondaparinux
- Mirogabalin
- Olodaterol
- Benzethonium Chloride
- Evobrutinib
- Temazepam
- Nalmefene
- Hyoscine Hydrobromide
- Nalidixic acid
- Icotinib
- Phenylbutyrate
- Taurine
- Pidotimod
- Mannitol
- Etamsylate
- Trenbolone Acetate
- Mazindol
- Fenproporex
- Denaverine
- Bemcentinib
- Amrubicin
- Polymyxin B
- Fenpiverinium
- Trifluridine
- Cyclopentolate
- Salacinol
- Dyclonine
- Talampicillin
- Halofuginone
- Campesterol
- Iodixanol
- Thiopental
- Cetrimide
- Acenocoumarol
- Dalargin
- Paromomycin
- Azafenidin
- Calcitonin Salmon
- Bisoctrizole
- Bambuterol
- Avobenzone
- Ensulizole
- Dihydroergocristine mesilate
- Nalbuphine
- Butylhydroxytoluene
- Cyanidin Chloride
- Saquinavir
- Monepantel
- Nitroxynil
- Fenbendazole
- Nitrendipine
- Tofisopam
- Colfosceril Palmitate
- Naloxegol
- Stanozolol
- Teduglutide
- Ixazomib
- Aclonifen
- Dihydroergotamine
- Xanthinol
- Daidzin
- Genistin
- Terlipressin
- Strychnine
- Mizolastine
- Oxyclozanide
- Cortisone
- Sonidegib
- Queuine
- Cedazuridine
- Guanabenz
- Arbutin
- Naftazone
- Brassicasterol
- Miglitol
- Diprophylline
- Mephenytoin
- Chlorphenesin
- Guanadrel
- Ethotoin
- Triflusal
- Panobinostat
- Benzphetamine
- Methcathinone
- Timoprazole
- Leminoprazole
- Saviprazole
- Disuprazole
- Deferoxamine
- Pamoic Acid
- Semaglutide
- Cloxazolam
- Etifoxine
- Gadopentetate
- Bacopaside
- Lactobionic Acid
- Lypressin
- Aprobarbital
- Pefloxacin
- Dichlorvos
- Semduramicin
- Aminophylline
- Saccharin
- Chlorthion
- Cyphenothrin
- Lactofen
- Oxychlordane
- Pamabrom
- Astemizole
- Caryophyllene oxide
- Quinalizarin
- Piribedil
- Possible Nitroso Standards
- Possible Nitroso Standards
- Pholcodine
- Degarelix
- Abaloparatide
- Liraglutide
- Stevioside
- Possible Nitroso Standards
- Spilanthol
- Octocrylene
- Arteether
- Taurodeoxycholic Acid
- Fluorescein
- Octinoxate
- Remimazolam
- Opipramol
- Metconazole
- Nitroscanate
- Iguratimod
- Thymosin
- Pramoxine
- Cefteram
- Deoxynivalenol
- Zearalenone
- Procaine
- Glucagon
- Calcium Saccharate
- Coumarin
- Mesterolone
- Glabridin
- Olsalazine
- Reproterol
- Parthenolide
- Yohimbine
- Tylosin
- Anhydrotetracycline
- Orientin
- Loprazolam
- Cephapirin
- Remifentanil
- Xipamide
- Articaine
- Betacarotene
- Gingerol
- Shogaol
- Dirithromycin
- Loxapine
- Bacampicillin
- Cerivastatin
- Vinpocetine
- Trelagliptin
- Flonicamid
- Narasin
- Teriparatide
- Spinosyn
- Dihydralazine
- Amidotrizoic Acid
- Lactoferrin
- Boswellic Acid
- Orotic Acid
- Tonibral
- Ecgonine
- Cenobamate
- Risdiplam
- Ataluren
- Brassinolide
- Miltefosine
- Vibegron
- Ecamsule
- Foscarnet
- Quinfamide
- Lofepramine
- Creatine
- Diflubenzuron
- Triflumuron
- Taurolithocholic Acid
- Inosine
- Capmatinib
- Caffeic Acid
- Myristic Acid
- Sobrerol
- Voxelotor
- Doripenem
- Biapenem
- Fimasartan
- Isopropyl Palmitate
- Flumioxazin
- Fludeoxyglucose
- Metacresol
- Triprolidine
- Vismodegib
- Dimethicone
- Aniracetam
- Nimorazole
- Chlorpyrifos
- Diazinon
- Elaiophylin
- Choline Chloride
- Treosulfan
- Prednisolone Pivalate
- Altretamine
- Lurbinectedin
- Tipiracil
- Abrocitinib
- Cytisine
- Bronopol
- Zotepine
- Sufentanil
- Fexuprazan
- Kanamycin
- Diphenoxylate
- Trehalose
- Tilmicosin
- Clorazepate
- Noscapine
- Sulfachlorpyridazine
- Menbutone
- Oclacitinib
- Mancozeb
- Epoxiconazole
- Amitraz
- Opicapone
- Hederacoside C
- Alfentanil
- Triazolam
- Avapritinib
- Pralsetinib
- Fostemsavir
- Oliceridine
- Nitrazepam
- Maribavir
- Ponesimod
- Melphalan Flufenamide
- Abametapir
- Asciminib
- Osilodrostat
- Berotralstat
- Tazemetostat
- Infigratinib
- Belzutifan
- Triheptanoin
- Tirbanibulin
- Lonafarnib
- Selumetinib
- Nifurtimox
- Ganaxolone
- Mavacamten
- Oteseconazole
- Futibatinib
- Omidenepag Isopropyl
- Daridorexant
- Mitapivat
- Trilaciclib
- Samidorphan
- Tetrazepam
- Pyrovalerone
- Fluoroestradiol (F-18)
- Flortaucipir (F-18)
- Mobocertinib
- Sotorasib
- Fosdenopterin
- Belumosudil
- Adagrasib
- Olutasidenib
- Lenacapavir
- Prazepam
- Deucravacitinib
- Pipradrol
- Deoxycholic Acid
- Allobarbital
- Pinazepam
- Nicotinic Acid
- Pethidine
- Allylprodine
- Phendimetrazine
- Alphameprodine
- Pemoline
- Alphamethadol
- Limaprost
- Oxazolam
- Nimetazepam
- Alphaprodine
- Oxfendazole
- Phenadoxone
- Phenampromide
- Fluazuron
- Phenomorphan
- Phenoperidine
- Piminodine
- Triclosan
- Dermatan Sulfate
- Candicidin
- Chondroitin Sulfate
- Piritramide
- Proheptazine
- Properidine
- Propiram
- Racemoramide
- Thebacon
- Thiofentanyl
- Eticyclidine
- Mescaline
- Psilocybine
- Sodium Stearyl Fumarate
- Matairesinol
- Pancuronium Bromide
- Rolicyclidine
- Mecloqualone
- Methamphetamine
- Phenmetrazine
- Secobarbital
- Zipeprol
- Butalbital
- Cathine
- Ampelopsin
- Camylofin
- Fenpyroximate
- Boldine
- Netilmicin
- Ethopabate
- Cyclobenzaprine
- Tedizolid
- Methaqualone
- Glutethimide
- Flurazepam
- Estazolam
- Methadone
- Brotizolam
- Clotiazepam
- Delorazepam
- Camazepam
- Fludiazepam
- Butorphanol
- Fluralaner
- Pentobarbital
- Pentazocine
- Ethchlorvynol
- Ethinamate
- Fencamfamin
- Mepartricin
- Oxethazaine
- Camphor
- Exenatide
- Cysteamine
- Bacitracin
- Ioversol
- Halazepam
- Haloxazolam
- Ketazolam
- Lefetamine
- Methyprylon
- Methylphenobarbital
- Mesocarb
- Cocaine
- Dextromoramide
- Meprobamate
- Mefenorex
- Medazepam
- Zaltoprofen
- Lormetazepam
- Amobarbital
- Anileridine
- Telaprevir
- Istradefylline
- Dextropropoxyphene
- Epiisopiloturine
- Pimozide
- Alitretinoin
- Diampromide
- Diethylthiambutene
- Norpipanone
- Normethadone
- Tetracosactide
- Sincalide
- Nalfurafine
- Chloroxylenol
- Meldonium
- Rigosertib
- Morniflumate
- Niflumic Acid
- Alfaxalone
- Dihydrocodeine
- Dihydromorphine
- Dimenoxadol
- Dimethylthiambutene
- Triclopyr
- Dipipanone
- Altiratinib
- Sitravatinib
- Fenetylline
- Sulthiame
- Chlorocresol
- Sertaconazole Nitrate
- Dactinomycin
- Nicarbazine
- Clorsulon
- Tiemonium Methylsulfate
- Thymol
- Toldimfos
- Carvone
- Procyanidin
- Lesinurad
- Epicatechin
- Flumequine
- Fluocortolone
- Voclosporin
- Metomidate
- Cefetamet
- Drotebanol
- Ethylmorphine
- Etonitazene
- Etoxeridine
- Diethylstilbestrol
- Furethidine
- Heroin
- Hydromorphone
- Isomethadone
- Ketobemidone
- Atezolizumab
- Pembrolizumab
- Propylthiouracil
- Testosterone Decanoate
- Levorphanol
- Succinylcholine
- Menadione
- Oxandrolone
- Ceftibuten
- Amylmetacresol
- Lomefloxacin
- Umckalin
- Disopyramide
- Isradipine
- Amphetamine Sulfate
- Mangiferin
- Azadirachtin
- Somatostatin
- Avacopan
- Mesotrione
- Setmelanotide
- Trifarotene
- Miramistin
- Arterolane
- Tirzepatide
- Sulbutiamine
- Chlorobutanol
- Pergolide
- Atipamezole
- Triclocarban
- Diphenidol
- Thalidomide
- Satranidazole
- Piperine
- Tartrazine
- Oxybenzone
- Teicoplanin
- Zanamivir
- Perazine
- Xanthone
- Hydrocodone
- Thebaine
- Benperidol
- Lysergide
- Bufotenine
- Propoxur
- Methomyl
- Cyhalothrin
- Imidacloprid
- Bifenthrin
- Difenoconazole
- Difenacoum
- Coumatetralyl
- Bromazepam
- Bromadiolone
- Afobazole
- Eugenol
- Cetilistat
- Alarelin
- Diuron
- Piperitone
- Tesofensine
- Hymecromone
- Nitrofural
- Lycorine
- Vestitol
- Medicarpin
- Homopterocarpin
- Altrenogest
- Xanthine
- Maltitol
- Pulegone
- Limonene
- Threonine
- Mitotane
- Ziprasidone
- Tandospirone
- Lomitapide
- Isomalt
- Pizotifen
- Gadoterate
- Pyronaridine
- Ziram
- Rhodamine 6G
- Indigo Carmine
- Agnuside
- Oteracil
- Tegafur
- Lomustine
- Alfadex
- Tyrothricin
- Terpin
- Tildipirosin
- Diosgenin
- Suxamethonium Chloride
- Elexacaftor
- Blonanserin--
- Azoxystrobin
- Cyproconazole
- Emrusolmin
- Izuforant
- Maralixibat
- Fenchone
- Lumacaftor
- Estetrol
- Triamcinolone Hexacetonide
- Glycyrrhizic acid
- Iobitridol
- Troxerutin
- Hypericin
- Iscotrizinol
- Catharanthine
- Metergoline
- Oxymorphone
- Ulinastatin
- Colistimethate
- Fenoxaprop
- Tripalmitin
- Oxymetholone
- Clothianidin
- Sulfametomidine
- Furagin
- Cannabigerol
- Clemizole
- Ethopropazine
- Naftopidil
- Temocapril
- Difamilast
- Rosavin
- Tetraxetan
- Astaxanthin
- Lecirelin
- Glycine
- Varespladib
- Ethylhexyl Triazone
- Ospemifene
- Moexipril
- Taurolidine
- Carteolol
- Vidarabine
- Bufuralol
- Dihydrotachysterol
- Laninamivir
- Hexoprenaline
- Tipepidine
- Nifursol
- Topotecan
- Miscellaneous-2
- Grazoprevir
- Pipamperone
- Cascaroside
- Norbelladine
- Taxifolin
- Andrographolide
- Glyphosate
- Benzetimide
- Gluconic Acid
- Trigonelline
- Exatecan
- Caricaxanthin
- Berberine
- Natamycin
- Teprenone
- Tilidine
- Arotinolol
- Lemborexant
- Seltorexant
- Zalunfiban
- Domiphen
- Sarecycline
- Virginiamycin
- all-rac-α-Tocopherol
- Histamine
- Belinostat
- Acetylcholine
- Azatadine
- Annamycin
- Phenelzine
- Atogepant
- Dexmethylphenidate
- Stigmastanol
- Sulfamerazine
- Melitracen
- Amprolium
- Antazoline
- Propiomazine
- Campestanol
- Dithranol
- Flutrimazole
- Frullanolide
- Galaxolide
- Alprenolol
- Apigenin
- Vosoritide
- Trofinetide
- Propamidine
- Josamycin Propionate
- Allantoin
- Dityrosine
- Reserpine
- Closantel
- Cromoglicic Acid
- Furazolidone
- Epigallocatechin Gallate
- Maduramicin
- Pasireotide
- Emedastine
- Chlorothiazide
- Cefquinome
- Danofloxacin
- Nafcillin
- Salinomycin
- Clofedanol
- Linaprazan
- Valethamate
- Methohexital
- Eupatilin
- Piroctone
- Diosmetin
- Epitalon
- Ipamorelin
- Zotatifin
- Chlorantraniliprole
- Cafedrine
- Clethodim
- Pyroxasulfone
- Revumenib
- Fruquintinib
- Octyl Gallate
- Apatinib
- Bibrocathol
- Oxeladin
- Auristatin
- Tolbutamide
- Landiolol
- Isocycloseram
- Pipecuronium Bromide
- Methacholine Chloride
- Nabilone
- Aleuritic Acid
- Voacamine
- Ibogaine
- Diazoxide
- Zastaprazan
- Procaterol
- Florasulam
- Picloram
- Mesosulfuron
- Idebenone
- Moxisylyte
- Resmetirom
- Gepirone
- Elacestrant
- Momelotinib
- Zuranolone
- Lotilaner
- Ritlecitinib
- Ambroxol
- Sotagliflozin
- Tilorone
- Fezolinetant
- Leniolisib
- Zavegepant
- Omaveloxolone
- Etrasimod
- Sparsentan
- Quizartinib
- Daprodustat
- Capivasertib
- Palovarotene
- Pirtobrutinib
- Rezafungin
- Nirogacestat
- Iptacopan
- Motixafortide
- Vamorolone
- Bexagliflozin
- Afoxolaner
- Rafoxanide
- Hydroxyethyl Salicylate
- Sulfamethazine
- Salidroside
- Pacritinib
- Tanespimycin
- Alvespimycin
- Anlotinib
- Etynodiol Diacetate
- Etizolam
- Pemafibrate
- Picloxydine
- Mangostin
- Brombuterol
- Cimaterol
- Cimbuterol
- Clenpenterol
- Pirenzepine
- Oxyphencyclimine
- Oxyphenbutazone
- Nylidrin
- Nicametate
- Ethylestrenol
- Mapenterol
- Carbodenafil
- Butropium Bromide
- Bucetin
- Acediasulfone
- Iomeprol
- Thonzylamine
- Telithromycin
- Telavancin
- Clerosterol
- Rolapitant
- Quinupristin
- Propylhexedrine
- Polythiazide
- Phenyltoloxamine
- Pafolacianine
- Padimate O
- Ingavirin
- Fenitrothion
- Levmetamfetamine
- Flutemetamol-(18F)
- Dipivefrin
- Florbetapir-(18F)
- Florbetaben-(18F)
- Exametazime
- Duvelisib
- Givinostat
- Vadadustat
- Danicopan
- Tovorafenib
- Mavorixafor
- Oxiconazole
- Rehmaionoside
- Turpinionoside
- Lauroside
- Aprotinin
- Phyllanthin
- Niranthin
- Quinapyramine
- Diclazuril
- Zoxamide
- Neoxanthin
- Scopoletin
- Cefovecin
- Angustifoline
- Anagyrine
- Tapinarof
- Xanomeline
- Cefotetan
- Sulfaguanidine
- Pterostilbene
- Evocalcet
- Camostat
- Neohesperidin Dihydrochalcone
- Asparagine
- Saroglitazar
- Aprocitentan
- Fusidic Acid
- Ferulic acid
- Enramycin
- Chloroprocaine
- Umbralisib
- Sarolaner
- Odevixibat
- Novaluron
- Etofenprox
- Artesunate
- Pyrroloquinoline Quinone
- Thioguanine
- Methyclothiazide
- Flavomycin
- Elafibranor
- Sofpironium Bromide
- Boscalid
- Melperone
- Desoxycorticosterone pivalate
- Justicidin
- Dotinurad
- Mexazolam
- Saflufenacil
- Naloxegol D5
- Halometasone
- Mephenesin
- Dapivirine
- Esaxerenone
- Ensifentrine
- Pentagastrin
- Perospirone
- Eldecalcitol
- Cyanidin Chloride
- Mavoglurant
- Diethylcarbamazine
- Elbasvir
- Tolimidone
- Flumethrin
- Tesamorelin
- Pralmorelin
- Clazuril
- Difelikefalin
- Thimerosal
- Methsuximide
- Vorasidenib
- Seladelpar
- Mephentermine
- Pangamic Acid
- Harmine
- Sitafloxacin
- Sulisobenzone
- Dioxybenzone
- Homosalate
- Meradimate
- Cefodizime
- Sovateltide
- Denosumab
- Sulfaquinoxaline
- Propiverine
- Cebranopadol
- Zilucoplan
- Ethionamide
- Vinorelbine
- Brivanib
- Lazertinib
- Tenoxicam
- Sulfalene
- Ripasudil
- Bismuth Subsalicylate
- Isocarboxazid
- Asunaprevir
- Isopropamide Iodide
- Sarpogrelate
- Amcinonide
- Retapamulin
- Alizapride
- Zidebactam
- Arimoclomol
- Levacetylleucine
- Squalene
- Porfiromycin
- Veratric acid
- Oxetorone
- Fenvalerate
- Vemurafenib
- Antroquinonol
- Segesterone Acetate
- Isometamidium Chloride
- Flomoxef
- Omethoate
- Amenamevir
- Tosylchloramide Sodium
- Osunprotafib
- Xylazine
- Chiglitazar
- Chloropyramine
- Ipidacrine
- Fluazinam
- Terfenadine
- Flurpiridaz-(18F)
- Inavolisib
- Acoramidis
- Sulfaphenazole
- Grapiprant
- Cefiderocol
- Gonadorelin
- Tiapride
- Vanzacaftor
- Thymalfasin
- Benzoxonium Chloride
- Benzgalantamine
- Acrinathrin
- Tetraconazole
- Fenoverine
- Acetorphine
- Pharmaceutical Reference Standards
- Benzethidine
- Betacetylmethadol
- Aminorex
- Mequitazine
- Sivelestat
- Gefapixant
- Crinecerfont
- Dovramilast
- Pharmaceutical Reference Standards
- Ethacizine
- Pharmaceutical Reference Standards
- Moricizine
- Emoxypine
- Linzagolix
- Ectoine
- Dembrexine
- Patiromer
- Orforglipron
- Acepromazine
- Pelitinib
- Amifostine
- Diloxanide
- Apraclonidine
- Benoxinate
- Danazol
- Mizoribine
- Ramoplanin
- Efrotomycin
- Lefamulin
- Taltirelin
- Valrubicin
- Calteridol
- Thiabendazole
- Buparvaquone