Search Results for " Furosemide "
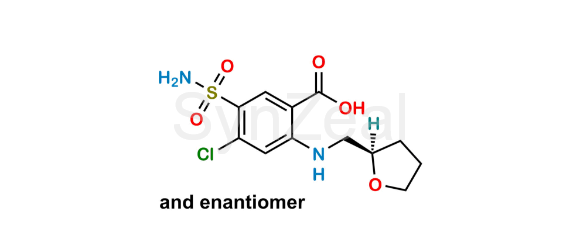
Furosemide EP Impurity F
| N° de SZ CAT: | SZ-F032007 |
| Número CAS | 4793-38-8 |
| Mol.F. | C12H15ClN2O5S |
| Peso Molecular | 334.8 |
| Status de Fatura | In Stock |
Nome Químico: 4-Chloro-5-sulfamoyl-2-[[[(2RS)-oxolan-2-yl]methyl]amino]benzoic acid (as per EP)
Sinônimo: Tetrahydro Furosemide
Shipping Temperature: Ambient
HSN Code: 38229010
Country of Origin: India
Sorrisos: O=C(O)C1=CC(S(=O)(N)=O)=C(Cl)C=C1NCC2OCCC2
Furosemide EP Impurity F is chemically 4-Chloro-5-sulfamoyl-2-[[[(2RS)-oxolan-2-yl]methyl]amino]benzoic acid (as per EP). It is also known as Tetrahydro Furosemide. Furosemide EP Impurity F is supplied with detailed characterization data compliant with regulatory guideline. Furosemide EP Impurity F can be used for the analytical method development, method validation (AMV), Quality Controlled (QC) application for Abbreviated New Drug Application (ANDA) or during commercial production of Furosemide.
The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. SynZeal products are for analytical purpose only and not for human use.
products.relatedproducts
Disclaimer
SynZeal product information given on this website is as per the existing understanding while publishing the details on website. The customer is responsible for assessing the accuracy of the information at the time of actual purchase.
SynZeal will update these details as per new development or finding in product specification without further noticed.

