OUR BLOGS
Check out our latest and upcoming Blogs.
Contact Us.png)
April 28 2022
Fluocinolone binds the glucocorticoid receptor, followed by translocation of the ligand-receptor complex
Read More ›

April 22 2022
Fesoterodine also known as toviaz, belongs to the class of organic compounds known as diphenylmethanes.
Read More ›
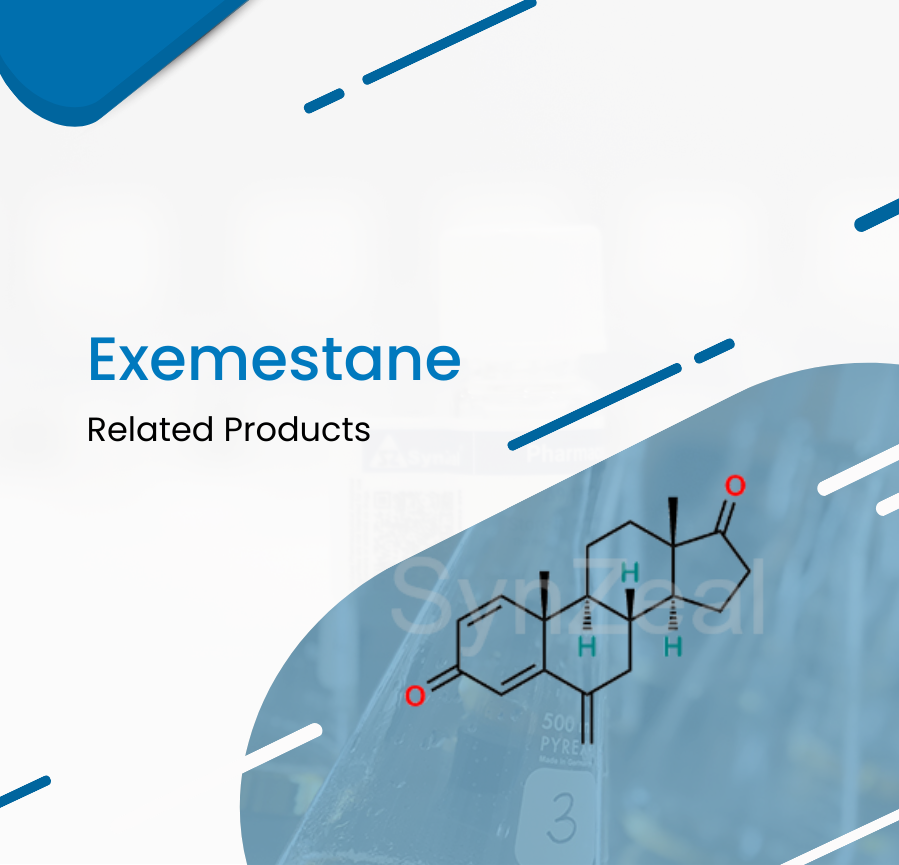
April 14 2022
Exemestane binds irreversibly to and inhibits the enzyme aromatase, thereby blocking the
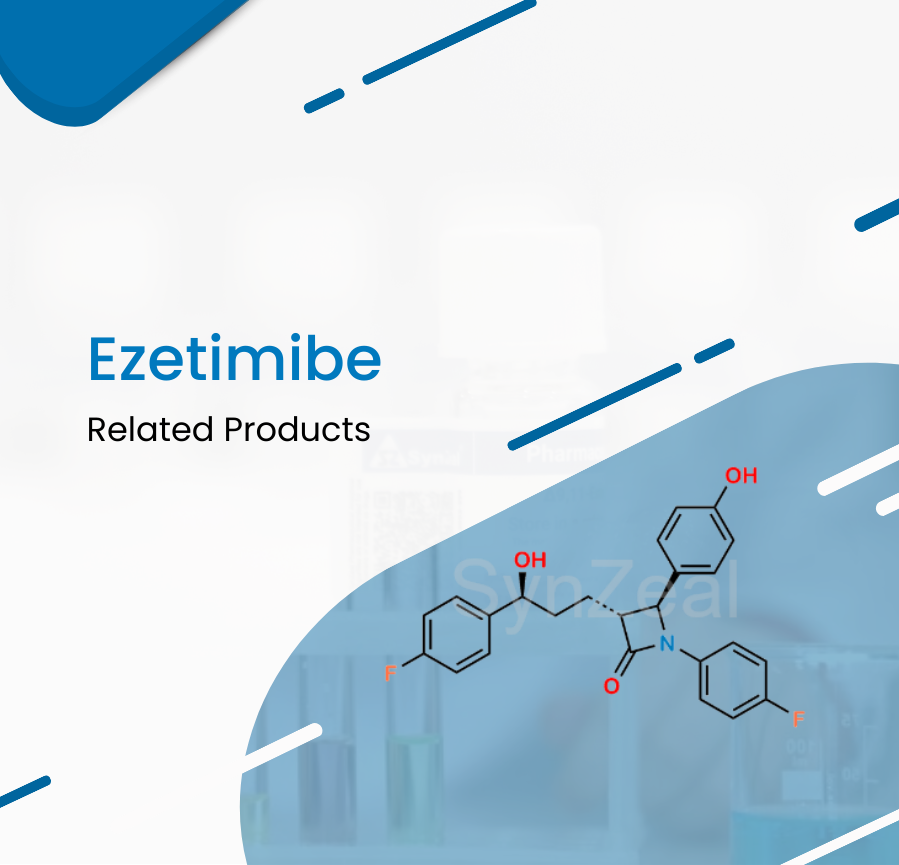
April 8 2022
Ezetimibe is a lipid-lowering compound that inhibits intestinal cholesterol and phytosterol absorption. Ezetimibe is used as an adjunctive therapy to a healthy diet t
Read More ›
March 31 2022
Etonogestrel molecule is a 3-ketodesogestrel or 19-nortestosterone which is a synthetic biologically active metabolite of progestin desogestrel.Etonogestrel is a drug
Read More ›